By: Hannes Schwandt

More than 2.5 million women are currently pregnant in the United States, and many are worried about the impact a coronavirus infection could have on mothers and the development of their unborn children. A large literature surrounding the fetal origins hypothesis has shown that pregnancy conditions not only impact the mother but can also impair the offspring (Almond and Currie 2011). Maternal influenza infections during pregnancy may induce premature birth (Currie and Schwandt 2013) and strong cases could even impair fetal development to the point where there are repercussions throughout adulthood (Schwandt 2019). In his seminal paper, Almond (2006) documented lower incomes and higher adult disability rates among the entire cohort of infants that was in-utero during the Spanish Flu pandemic.
Will the children born during the coronavirus pandemic suffer the same fate as those born during the 1918/19 Spanish Flu pandemic? While at this moment nobody can say with certainty whether maternal coronavirus infections impair fetal development, the existing evidence allows for cautious optimism. The rate of reported COVID-19 deaths among pregnant mothers is low (Hantoushzadeh 2020) and while infections occur in pregnant mothers, most are mild or remain asymptomatic (Sutton et al. 2020; Luo and Yin 2020). Children of mothers with COVID-19 during pregnancy are born without detectable impairments and typically test negative for the virus (Chen et al. 2020; Yu et al. 2020).
But how did pregnant mothers fare during the Spanish Flu pandemic in comparison to the current pandemic? Could in-utero exposure to maternal respiratory disease have negative long-term consequences that are not immediately visible at birth?
The current pandemic is often compared to the Spanish Flu pandemic, but it is important to keep in mind that the 1918 influenza virus had a very different, more devastating impact – particularly for pregnant mothers. Figure 1 shows the age profiles for both COVID-19 mortality in New York City and Spanish Flu mortality in Philadelphia, one of the most severely hit cities during the Spanish Flu pandemic (Markel 2007). Both mortality rates are shown per 100,000 people, but are plotted on different y-axes since COVID-19 deaths rates are likely to continue increasing in the ongoing pandemic.
Figure 1: COVID-19 mortality (in NYC) and Spanish Flu mortality (in Philadelphia) by age and gender
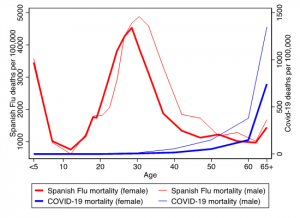
Notes: Data on Covid-19 death counts for New York City, 2/1/2020-5/20/2020 (CDC, 2020), combined with population estimates for 2017 (NYSDH, 2017). Data on Spanish Flu mortality from Rogers (1920).
COVID-19 mortality increases exponentially with age, with extremely low death rates among infants, children, and young adults (these numbers include recent cases of multi-system inflammatory syndrome; Schroeder 2020). Mortality is particularly low for young women. Among a total of 16,493 COVID-19 deaths in New York City (between 2/1/2020 and 5/20/2020), not a single female fatality occurred below age 25. For women between the ages of 25 and 34, only five out of every 100,000 have died —and this figure is far lower than the rate for women 65 or older, where the data show more than 740 deaths per 100,000.
For the Spanish Flu, the age profile of deaths looked dramatically different. Fatalities were highly concentrated among infants and young adults. Among women, the majority of deaths occurred in the fertile age range and mortality was especially high among pregnant women (Almond 2006). For every death there were many cases of severe illness, with recorded stillbirths more than doubling during the pandemic (Rogers, 1920).
In other words, pregnant mothers who survived the Spanish Flu experienced extreme levels of sickness in response to influenza infections during the pandemic. This strong impact could not be more different from the current experience with coronavirus infections, which often go largely unnoticed in pregnant mothers.
This brings us to the last question in assessing the relative risks between the two pandemics for a developing fetus: Are mothers’ mild responses to coronavirus infections good or bad for an infant in utero? Strong maternal reactions observed for influenza infections may drain the mother’s body, but they could be an efficient response, e.g., to protect the fetus from contracting the virus itself.
It turns out that the fetus does not usually get infected if the pregnant mother contracts the influenza virus or the coronavirus (the virus does not pass the placenta). It is the strong maternal response to the influenza virus that impairs fetal development. The strong immune system response can lead to broad inflammations in the pregnant body (McDade 2012) which fight not only the virus but also damage healthy tissue such as the placenta.
Pregnant women’s mild immune system response to COVID-19 is, therefore, a hopeful sign. Strong inflammations are also hypothesized to turn the mother’s immune system itself against the fetus. The father’s genes in the fetus are foreign to the mother’s body and are only tolerated due to the mediation of helper cells surrounding the placenta (Trowsdale and Betz 2006). A strong inflammatory response can compromise the functionality of these helper cells. As a result, the fetus might not only suffer from the inflammation itself, but this inflammation can cause the mother’s immune system to harm the fetus. It remains unclear why pregnancy makes women respond so strongly to the influenza virus (Kay et al., 2014), but it happens both for pandemics and seasonal influenza. And the resulting infections have similar negative impacts on the offspring – at birth and into adulthood (Almond 2006, Creanga et al. 2009; Currie and Schwandt 2013, Schwandt 2019).
Should our reaction be cautious hope and stress relief? It is important to emphasize that our understanding about the threats of the coronavirus is still far from complete. We are learning from new research findings on a daily basis. But there’s good news from what we know so far: the coronavirus seems to impact pregnant women less than influenza does. Maternal influenza infections typically activate immune system responses, which have been shown to impair fetal development, but this does not seem to be happening here. Of course, it is still possible that other impairments in the offspring may appear later, even if they are not seen at birth. And only time will tell whether infants exposed to COVID-19 during the first or second trimester will show signs of impairment at birth. But, so far, the existing evidence allows for cautious hope that pregnant mothers and their offspring will fare much better than they did during the last devastating global pandemic at the turn of the 20th century.
This is not to say that pregnant mothers and young children are entirely unaffected by the current pandemic. Food insecurity is on the rise (Schanzenbach and Pitts 2020), and many young families face financial stress as unemployment is soaring to unprecedented levels. Nutritional, economic, and psychological stress during pregnancy is bad for the fetus – it increases the risk of poor health at birth and negative long-term consequences (Aizer et al. 2016; Almond et al. 2011; Persson and Rossin-Slater 2018). Furthermore, we do not want to add unfounded anxieties about COVID-19 infections to mothers’ list of worries—and fewer worries might help their developing infants most.
References:
Aizer, A., Stroud, L. and Buka, S., 2016. Maternal stress and child outcomes: Evidence from siblings. Journal of Human Resources, 51(3), pp.523-555.
Almond, D., 2006. Is the 1918 influenza pandemic over? Long-term effects of in utero influenza exposure in the post-1940 US population. Journal of Political Economy, 114(4), pp.672-712.
Almond, D., Hoynes, H.W. and Schanzenbach, D.W., 2011. Inside the war on poverty: The impact of food stamps on birth outcomes. Review of Economics and Statistics, 93(2), pp.387-403.
CDC, National Center for Health Statistics. (2020). Provisional COVID-19 Death Counts by Sex, Age and State. Retrieved on 5/22/2020 from CDC website: https://data.cdc.gov/NCHS/Provisional-COVID-19-Death-Counts-by-Sex-Age-and-S/9bhg-hcku
Currie, J. and Almond, D., 2011. Human capital development before age five. In Handbook of Labor Economics (Vol. 4, pp. 1315-1486). Elsevier.Chen, L., Li, Q., Zheng, D., Jiang, H., et al. (2020). Clinical Characteristics of Pregnant Women with Covid-19 in Wuhan, China. New England Journal of Medicine.
Creanga, A. A., Kamimoto, L., Newsome, K., et al. (2011). Seasonal and 2009 pandemic influenza A (H1N1) virus infection during pregnancy: a population-based study of hospitalized cases. American journal of obstetrics and gynecology, 204(6), S38-S45.
Currie, J. and Schwandt, H., 2013. Within-mother analysis of seasonal patterns in health at birth. Proceedings of the National Academy of Sciences, 110(30), pp.12265-12270.
Hantoushzadeh, S., Shamshirsaz, A.A., Aleyasin, A., Seferovic, M.D., Aski, S.K., Arian, S.E., Pooransari, P., Ghotbizadeh, F., Aalipour, S., Soleimani, Z. and Naemi, M., 2020. Maternal Death Due to COVID-19 Disease. American Journal of Obstetrics and Gynecology.
Luo, Y., & Yin, K. (2020). Management of pregnant women infected with COVID-19. The Lancet, 20(5), 513-514.
Markel, H., Lipman, H. B., Navarro, J. A., Sloan, A., Michalsen, J. R., Stern, A. M., & Cetron, M. S. (2007). Nonpharmaceutical interventions implemented by US cities during the 1918-1919 influenza pandemic. JAMA, 298(6), 644-654.
McDade, T.W., 2012. Early environments and the ecology of inflammation. Proceedings of the National Academy of Sciences of the United States of America, 109, p.17281.
NYSDH. (2017). Vital Statistics of New York State 2017 (Table 1: Estimated Population by Age, Sex and Region, New York State – 2017). Retrieved from https://www.health.ny.gov/statistics/vital_statistics/2017/table01.htm
Persson, P. and Rossin-Slater, M., 2018. Family ruptures, stress, and the mental health of the next generation. American Economic Review, 108(4-5), pp.1214-52.
Rogers, S. L. (1920). Special tables of mortality from influenza and pneumonia in Indiana, Kansas, and Philadelphia, PA. Department of Commerce, Bureau of the Census
Schanzenbach, D. and A. Pitts (2020) Estimates of Food Insecurity During the COVID-19 Crisis: Results from the COVID Impact Survey, Week 2 (May4–10, 2020). Northwestern University Institute for Policy Research Report.
Schroeder, A.R., Wilson, K.M. and Ralston, S.L., 2020. COVID-19 and Kawasaki disease: finding the signal in the noise. Hospital Pediatrics.
Schwandt, H., (2019). The lasting legacy of seasonal influenza: In-utero exposure and labor market outcomes. Working paper.
Sutton, D., Fuchs, K., D’alton, M., & Goffman, D. (2020). Universal screening for SARS-CoV-2 in women admitted for delivery. New England Journal of Medicine.
Trowsdale, J. and A. Betz. 2006. Mother’s little helpers: Mechanisms of maternal-fetal tolerance. Nature Immunology 7(3): 241–6.
Yu, N., Li, W., Kang, Q., Xiong, Z., Wang, S., Lin, X., Liu, Y., Xiao, J., Liu, H., Deng, D., Chen, S., Zeng, W., Feng, L., & Wu, J. (2020). Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. The Lancet, 20(5), 559-564.
Hannes Schwandt is an Assistant Professor of Education and Social Policy at Northwestern University.
